|
Chapter 29 : Organic chemicals
Notes. 1. Except where the context otherwise requires, the headings of this Chapter apply only to : (a) Separate chemically defined organic compounds, whether or not containing impurities; (b) Mixtures of two or more isomers of the same organic compound (whether or not containing impurities), except mixtures of acyclic hydrocarbon isomers (other than stereoisomers), whether or not saturated (Chapter 27); (c) The products of headings 29.36 to 29.39 or the sugar ethers, sugar acetals and sugar esters, and their salts, of heading 29.40, or the products of heading 29.41, whether or not chemically defined; (d) The products mentioned in (a), (b) or (c) above dissolved in water; (e) The products mentioned in (a), (b) or (c) above dissolved in other solvents provided that the solution constitutes a normal and necessary method of putting up these products adopted solely for reasons of safety or for transport and that the solvent does not render the product particularly suitable for specific use rather than for general use; (f) The products mentioned in (a), (b), (c), (d) or (e) above with an added stabiliser (including an anti-caking agent) necessary for their preservation or transport; (g) The products mentioned in (a), (b), (c), (d), (e) or (f) above with an added anti-dusting agent or a colouring or odoriferous substance added to facilitate their identification or for safety reasons, provided that the additions do not render the product particularly suitable for specific use rather than for general use; (h) The following products, diluted to standard strengths, for the production of azo dyes : diazonium salts, couplers used for these salts and diazotisable amines and their salts. 2. This Chapter does not cover : (a) Goods of heading 15.04 or crude glycerol of heading 15.20; (b) Ethyl alcohol (heading 22.07 or 22.08); (c) Methane or propane (heading 27.11); (d) The compounds of carbon mentioned in Note 2 to Chapter 28; (e) Immunological products of heading 30.02. (f) Urea (heading 31.02 or 31.05); (g) Colouring matter of vegetable or animal origin (heading 32.03), synthetic organic colouring matter, synthetic organic products of a kind used as fluorescent brightening agents or as luminophores (heading 32.04) or dyes or other colouring matter put up in forms or packings for retail sale (heading 32.12); (h) Enzymes (heading 35.07); (ij) Metaldehyde, hexamethylenetetramine or similar substances, put up in forms (for example, tablets, sticks or similar forms) for use as fuels, or liquid or liquefied-gas fuels in containers of a kind used for filling or refilling cigarette or similar lighters and of a capacity not exceeding 300 cm3 (heading 36.06); (k) Products put up as charges for fire-extinguishers or put up in fire-extinguishing grenades, of heading 38.13; ink removers put up in packings for retail sale, of heading 38.24; or (l) Optical elements, for example, of ethylenediamine tartrate (heading 90.01). 3. Goods which could be included in two or more of the headings of this Chapter are to be classified in that one of those headings which occurs last in numerical order. 4. In headings 29.04 to 29.06, 29.08 to 29.11 and 29.13 to 29.20, any reference to halogenated, sulphonated, nitrated or nitrosated derivatives includes a reference to compound derivatives, such as sulphohalogenated, nitrohalogenated, nitrosulphonated or nitrosulphohalogenated derivatives.
Nitro or nitroso groups are not to be taken as "nitrogen-functions" for the purpose of heading 29.29.
For the purposes of headings 29.11, 29.12, 29.14, 29.18 and 29.22, "oxygen-function" is to be restricted to the functions (the characteristic organic oxygen-containing groups) referred to in headings 29.05 to 29.20. 5. (A) The esters of acid-function organic compounds of sub-Chapters I to VII with organic compounds of these sub-Chapters are to be classified with that compound which is classified in the heading which occurs last in numerical order in these sub-Chapters. (B) Esters of ethyl alcohol with acid-function organic compounds of sub-Chapters I to VII are to be classified in the same heading as the corresponding acid-function compounds. (C) Subject to Note 1 to Section VI and Note 2 to Chapter 28 : (1) Inorganic salts of organic compounds such as acid-, phenol- or enol-function compounds or organic bases, of sub-Chapters I to X or heading 29.42, are to be classified in the heading appropriate to the organic compound; (2) Salts formed between organic compounds of sub-Chapters I to X or heading 29.42 are to be classified in the heading appropriate to the base or to the acid (including phenol- or enol-function compounds) from which they are formed, whichever occurs last in numerical order in the Chapter; and (3) Co-ordination compounds, other than products classifiable in sub-Chapter XI or heading 29.41, are to be classified in the heading which occurs last in numerical order in Chapter 29, among those appropriate to the fragments formed by "cleaving" of all metal bonds, other than metal-carbon bonds. (D) Metal alcoholates are to be classified in the same heading as the corresponding alcohols except in the case of ethanol (heading 29.05). (E) Halides of carboxylic acids are to be classified in the same heading as the corresponding acids. 6. The compounds of headings 29.30 and 29.31 are organic compounds the molecules of which contain, in addition to atoms of hydrogen, oxygen or nitrogen, atoms of other non-metals or of metals (such as sulphur, arsenic or lead) directly linked to carbon atoms.
Heading 29.30 (organo-sulphur compounds) and heading 29.31 (other organo-inorganic compounds) do not include sulphonated or halogenated derivatives (including compound derivatives) which, apart from hydrogen, oxygen and nitrogen, only have directly linked to carbon the atoms of sulphur or of a halogen which give them their nature of sulphonated or halogenated derivatives (or compound derivatives). 7. Headings 29.32, 29.33 and 29.34 do not include epoxides with a three-membered ring, ketone peroxides, cyclic polymers of aldehydes or of thioaldehydes, anhydrides of polybasic carboxylic acids, cyclic esters of polyhydric alcohols or phenols with polybasic acids, or imides of polybasic acids.
These provisions apply only when the ring-position hetero-atoms are those resulting solely from the cyclising function or functions here listed. 8. For the purposes of heading 29.37 : (a) the term "hormones" includes hormone-releasing or hormone-stimulating factors, hormone inhibitors and hormone antagonists (anti-hormones); (b) the expression "used primarily as hormones" applies not only to hormone derivatives and structural analogues used primarily for their hormonal effect, but also to those derivatives and structural analogues used primarily as intermediates in the synthesis of products of this heading. Subheading Notes. 1. Within any one heading of this Chapter, derivatives of a chemical compound (or group of chemical compounds) are to be classified in the same subheading as that compound (or group of compounds) provided that they are not more specifically covered by any other subheading and that there is no residual subheading named "Other" in the series of subheadings concerned. 2. Note 3 to Chapter 29 does not apply to the subheadings of this Chapter. GENERAL
As a general rule, this Chapter is restricted to separate chemically defined compounds, subject to the provisions of Note 1 to the Chapter. (A) Chemically defined compounds (Chapter Note 1)
A separate chemically defined compound is a substance which consists of one molecular species (e.g., covalent or ionic) whose composition is defined by a constant ratio of elements and can be represented by a definitive structural diagram. In a crystal lattice, the molecular species corresponds to the repeating unit cell.
Separate chemically defined compounds containing other substances deliberately added during or after theirmanufacture (including purification) are excluded from this Chapter. Accordingly, a product consisting of saccharin mixed with lactose, for example, to render the product suitable for use as a sweetening agent is excluded (see Explanatory Note to heading 29.25).
The separate chemically defined compounds of this Chapter may contain impurities (Note 1 (a)). An exception to this rule is created by the wording of heading 29.40 which, with regard to sugars, restricts the scope of the heading to chemically pure sugars.
The term "impurities" applies exclusively to substances whose presence in the single chemical compound results solely and directly from the manufacturing process (including purification). These substances may result from any of the factors involved in the process and are principally the following : (a) Unconverted starting materials. (b) Impurities present in the starting materials. (c) Reagents used in the manufacturing process (including purification). (d) By-products.
It should be noted, however, that such substances are not in all cases regarded as "impurities" permitted under Note 1 (a). When such substances are deliberately left in the product with a view to rendering it particularly suitable for specific use rather than for general use, they are not regarded as permissible impurities. For example, a product consisting of methyl acetate with methanol deliberately left in with a view to improving its suitability as a solvent is excluded (heading 38.14). For certain compounds (e.g., ethane, benzene, phenol, pyridine), there are specific purity criteria, indicated in Explanatory Notes to headings 29.01, 29.02, 29.07 and 29.33.
The separate chemically defined compounds of this Chapter may be dissolved in water. Subject to the samequalifications as those set out in the General Explanatory Note to Chapter 28, this Chapter also includes non-aqueous solutions and also compounds (or their solutions) with added stabilisers, antidusting agents or colouring substances. For example, styrene inhibited with tertiary butylcatechol remains classified in heading 29.02. The provisions in the General Explanatory Note to Chapter 28 concerning the addition of stabilisers, antidusting agents and colouring substances apply, mutatis mutandis, to the chemical compounds of this Chapter. Subject to the same qualifications as those made in respect of colouring substances, these compounds may also contain added odoriferous substances (e.g., bromomethane of heading 29.03 to which small quantities of chloropicrin have been added).
This Chapter further includes, whether or not they contain impurities, mixtures of isomers of the same organic compound. This provision applies only to mixtures of compounds having the same chemical function (or functions) and which either coexist in their natural form or are obtained simultaneously in the course of the same synthesis. Mixtures of acyclic hydrocarbon isomers (other than stereoisomers), whether or not saturated, are, however, excluded (Chapter 27). (B) Distinction between the compounds of Chapter 28 and those of Chapter 29
Organic compounds of precious metals, radioactive elements, isotopes, rare-earth metals, yttrium and scandium, and the other compounds containing carbon listed in Part (B) of the General Explanatory Note to Chapter 28 are excluded from Chapter 29 (see Note 1 to Section VI and Note 2 to Chapter 28).
Organo-inorganic compounds, other than those listed in Note 2 to Chapter 28, fall in Chapter 29. (C) Products which remain classified in Chapter 29,
even when they are not separate chemically defined compounds
There are certain exceptions to the rule that Chapter 29 is limited to separate chemically defined compounds. These exceptions include the following products :
Heading 29.09 - Ketone peroxides.
Heading 29.12 - Cyclic polymers of aldehydes; paraformaldehyde.
Heading 29.19 - Lactophosphates.
Heading 29.23 - Lecithins and other phosphoaminolipids.
Heading 29.34 - Nucleic acids and their salts.
Heading 29.36 - Provitamins and vitamins (including concentrates and intermixtures), whether or not in a solvent.
Heading 29.37 - Hormones.
Heading 29.38 - Glycosides and their derivatives.
Heading 29.39 - Vegetable alkaloids and their derivatives.
Heading 29.40 - Sugar ethers, sugar acetals and sugar esters, and their salts.
Heading 29.41 - Antibiotics.
This Chapter also includes diazonium salts (see Part (A) of Explanatory Note to heading 29.27), couplers used for these salts and diazotisable amines and their salts, diluted with e.g., neutral salts to standard strengths. These are designed for the production of azo dyes. They may be solid or liquid.
This Chapter further includes pegylated (polyethylene glycol (or PEGs) polymers) derivatives of products of headings 29.36 to 29.39 and 29.41. For these products, a pegylated derivative remains classified in the same heading as its non-pegylated form. However, pegylated derivatives of products of all other headings of Chapter 29 are excluded (generally heading 39.07). (D) Exclusion from Chapter 29 of certain separate chemically defined organic compounds (Chapter Note 2) (1) Certain separate chemically defined organic compounds are always excluded from Chapter 29, even when they are pure. In addition to those which fall in Chapter 28 (see Part (B) of the General Explanatory Note to that Chapter), examples of compounds of this group are : (a) Sucrose (heading 17.01); lactose, maltose, glucose and fructose (heading 17.02). (b) Ethyl alcohol (heading 22.07 or 22.08). (c) Methane and propane (heading 27.11). (d) Immunological products (heading 30.02). (e) Urea (heading 31.02 or 31.05). (f) Colouring matter of animal or vegetable origin (e.g., chlorophyll) (heading 32.03). (g) Synthetic organic colouring matter (including pigments), and synthetic organic products of a kind used as fluorescent brightening agents (e.g., certain stilbene derivatives) (heading 32.04). (2) Certain other separate chemically defined organic products, which would otherwise have been classified in Chapter 29, may be excluded when put up in certain forms, or if they have been subjected to certain treatments which leave their chemical composition unchanged. Examples are : (a) Products for therapeutic or prophylactic uses, put up in measured doses or in forms or in packings for retail sale (heading 30.04). (b) Products of a kind used as luminophores (e.g., salicylaldazine) which have been treated to render them luminescent (heading 32.04). (c) Dyes and other colouring matter put up in forms or packings for retail sale (heading 32.12). (d) Perfumery, cosmetic or toilet preparations (e.g., acetone), put up in packings for retail sale for such use (headings 33.03 to 33.07). (e) Products suitable for use as glues or adhesives, put up for retail sale as glues or adhesives, not exceeding a net weight of 1 kg (heading 35.06). (f) Solid fuels (e.g., metaldehyde, hexamethylenetetramine) put up in forms for use as fuels, and liquid or liquefied fuels (e.g., liquid butane) in containers of a kind used for filling or refilling cigarette or similar lighters and of a capacity not exceeding 300 cm3 (heading 36.06). (g) Hydroquinone and other unmixed products for photographic uses, put up in measured portions or put up for retail sale in a form ready for photographic use (heading 37.07). (h) Disinfectants, insecticides, etc., put up as described in heading 38.08. (ij) Products (e.g., carbon tetrachloride) put up as charges for fire-extinguishers or put up in fire-extinguishing grenades (heading 38.13). (k) Ink removers (e.g., chloramines of heading 29.35 dissolved in water) put up in packings for retail sale (heading 38.24). (l) Optical elements (e.g., ethylenediamine tartrate) (heading 90.01). (E) Products potentially classifiable in two or more headings of Chapter 29 (Chapter Note 3)
Such products are to be classified in the heading placed last in numerical order amongst those which could be applied. For example, ascorbic acid could be regarded as a lactone (heading 29.32) or as a vitamin (heading 29.36); it should therefore be classified in heading 29.36. For the same reason, allylestrenol which is a cyclic alcohol (heading 29.06) but also a steroid with unmodified gonane structure, used primarily for its hormone function (heading 29.37), should fall in heading 29.37.
It should, however, be noted that the last phrase of the text of heading 29.40 specifically excludes the products of headings 29.37, 29.38 and 29.39. (F) Halogenated, sulphonated, nitrated or nitrosated derivatives and combinations thereof (Chapter Note 4)
Certain headings of Chapter 29 include references to halogenated, sulphonated, nitrated or nitrosated derivatives. Such references include compound derivatives, for example, sulphohalogenated, nitrohalogenated, nitrosulphonated, nitrosulphohalogenated, etc., derivatives.
Nitro and nitroso groups are not to be taken as nitrogen-functions for the purpose of heading 29.29.
The halogenated, sulphonated, nitrated and nitrosated derivatives are formed by substitution of one or more hydrogen atoms in the parent compound by one or more halogens, sulpho (‑SO3H), nitro (-NO2) or nitroso (-NO) groups or by any combination thereof. Any functional group (e.g., aldehyde, carboxylic acid, amine) taken into consideration for classification should remain intact in such derivatives. (G) Classification of esters, salts, co-ordination compounds and certain halides (Chapter Note 5) (1) Esters.
The esters of acid-function organic compounds of sub-Chapters I to VII with organic compounds of these sub-Chapters are to be classified with that compound which is classified in the heading which occurs last in numerical order in these sub-Chapters.
Examples : (a) Diethylene glycol acetate (ester formed by the reaction of acetic acid of heading 29.15 with diethylene glycol of heading 29.09) - Heading 29.15 (b) Methyl benzenesulphonate (ester formed by the reaction of benzene-sulphonic acid
of heading 29.04 with methyl alcohol of heading 29.05) - Heading 29.05 (c) Butyl hydrogenphthalate (ester of a polycarboxylic acid where the hydrogen of only one (COOH) group has been substituted) - Heading 29.17 (d) Butyl phthalyl butyl glycollate (ester formed by the reaction of phthalic acid
of heading 29.17 and glycollic acid of heading 29.18 with butyl alcohol of heading 29.05) Heading 29.18
This rule cannot be applied to the esters of such acid-function compounds with ethyl alcohol since this compound is not classified in Chapter 29. Such esters are to be classified with the acid-function compounds from which they are derived.
Example :
Ethyl acetate (ester formed by the reaction of acetic acid of heading 29.15 with ethyl alcohol) Heading 29.15
It should further be noted that sugar esters and their salts are classified in heading 29.40. (2) Salts.
Subject to Note 1 to Section VI and Note 2 to Chapter 28 : (a) Inorganic salts of organic compounds such as acid-, phenol- or enol-function compounds or organic bases, of sub-Chapters I to X or heading 29.42, are to be classified in the heading appropriate to the organic compound.
These salts may be formed by the reaction of : (ⅰ) Acid-, phenol- or enol-function organic compounds with inorganic bases.
Example :
Sodium methoxybenzoate (salt formed by the reaction of methoxy-benzoic acid of heading 29.18 with sodium hydroxide) - Heading 29.18
Salts of this category may also be formed by the reaction of acid esters of the type referred to above with inorganic bases.
Example :
n-Butyl copper phthalate (salt formed by the reaction of butyl hydrogen phthalate of heading 29.17 with copper hydroxide) - Heading 29.17 or (ⅱ) Organic bases with inorganic acids.
Example :
Diethylamine hydrochloride (salt formed by the reaction of diethylamine of heading 29.21 with hydrochloric acid of heading 28.06) - Heading 29.21 (b) Salts formed between organic compounds of sub-Chapters I to X or heading 29.42 are to be classified in the heading appropriate to the base or to the acid (including phenol- or enol-function compounds) from which they are formed, whichever occurs last in numerical order in the Chapter.
Examples : (ⅰ) Aniline acetate (salt formed by the reaction of acetic acid of heading 29.15 with aniline of heading 29.21) - Heading 29.21 (ⅱ) Methylamine phenoxyacetate (salt formed by the reaction of methylamine of heading 29.21 with phenoxyacetic acid of heading 29.18) - Heading 29.21 (3) Co-ordination compounds.
Metal co-ordination compounds generally include all the types, whether or not charged, in which a metal is bound to several atoms (generally 2 to 9 atoms) made available by one or more ligands. The skeletal geometry formed by the metal and the atoms which are bound to it as well as the number of metal links are generally characteristic for a given metal.
Co-ordination compounds, other than products classifiable in sub-Chapter XI or in heading 29.41, should be considered as being fragmented by "cleaving" of all metal bonds, apart from metal-carbon bonds, and should be classified according to the fragment (regarded as a real compound for classification purposes) falling in Chapter 29, in the heading occurring last in numerical order.
For the purposes of Note 5 (C) (3) to this Chapter, the term "fragments" covers the ligands and the part(s) containing the metal-carbon bond that have resulted from the cleavage.
Examples are shown below :
Potassium trioxalatoferrate (Ⅲ) is classifiable in the heading in which the oxalic acid falls (heading 29.17), corresponding to the fragment obtained after cleaving of the metal bonds. 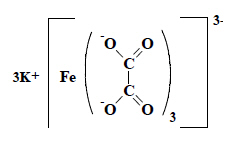 |
Ferrocholinate (INN) is classifiable in the heading covering choline (heading 29.23), which is classified in the heading occuring last in numerical order, rather than in the heading for citric acid corresponding to the other fragment that can be taken into account for classification purposes.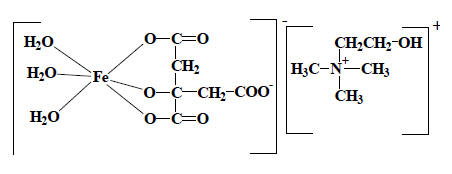 |
Budotitane (INN) : After cleaving of the metal bonds, two fragments are obtained, one corresponding to ethanol (Chapter 22), the other to benzoylacetone (and its enol-function) classified in heading 29.14. Budotitane (INN) should therefore be classified in heading 29.14.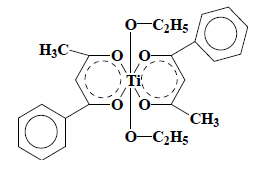 |
(4) Halides of carboxylic acids.
Such halides are classified in the same heading as the corresponding acids. For example, isobutyryl chloride is classified (like the isobutyric acid to which it corresponds) in heading 29.15. (H) Classification in headings 29.32, 29.33 and 29.34 (Chapter Note 7)
Headings 29.32, 29.33 and 29.34 do not include epoxides with a three-membered ring, ketone peroxides, cyclic polymers of aldehydes or of thioaldehydes, anhydrides of polybasic carboxylic acids, cyclic esters of polyhydric alcohols or phenols with polybasic acids, or imides of polybasic acids, if the ring-position hetero-atoms are those resulting solely from the cyclising function or functions here listed.
If, in addition to functions listed in the first sentence of Note 7 to Chapter 29, there are other ring-position hetero-atoms present in the structure, the classification should be carried out with reference to all the cyclising functions present. Thus, for example, anaxirone (INN) and pradefovir (INN) should be classified in heading 29.34 as heterocyclic compounds with two or more different hetero-atoms and not in heading 29.33 as heterocyclic compounds with nitrogen hetero-atoms only. 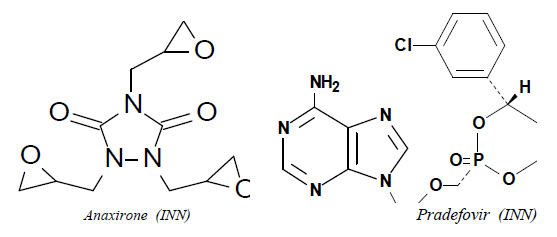 |
(IJ) Classification of derivatives
The classification of derivatives of chemical compounds at heading level is to be determined by application of the General Interpretative Rules. Note 3 to this Chapter applies when a derivative is potentially classifiable in two or more headings.
Within any one heading of this Chapter, derivatives are to be classified by application of Subheading Note 1. (K) Fused ring systems
A fused ring system is one in which there are at least two rings which have one, and only one, common bond and have two, and only two, atoms in common.
Fused ring systems appear in the molecules of polycyclic compounds (e.g., polycyclic hydrocarbons, heterocyclic compounds) in which two cyclic rings are joined by a common side involving two adjacent atoms. Examples are shown below :
In complex ring systems, fusion can take place to more than one side of any particular ring. Polycyclic compounds in which two rings have two, and only two, atoms in common are said to be" ortho-fused". On the other hand, polycyclic compounds in which one ring contains two, and only two, atoms in common with each of the two or more rings of a contiguous series of rings are said to be" ortho- and peri-fused". These two different types of fused ring systems are illustrated by the following examples :
On the other hand, the following is an example of a bridged (not fused) quinoline :
|